In the same section
-
Share this page
Device for cold plasma treatment [Technology offer]
The technology in a nutshell
New device and treatment using cold atmospheric plasma for ablation of the duodenal lining or regeneration of the mucosa.
State of the art
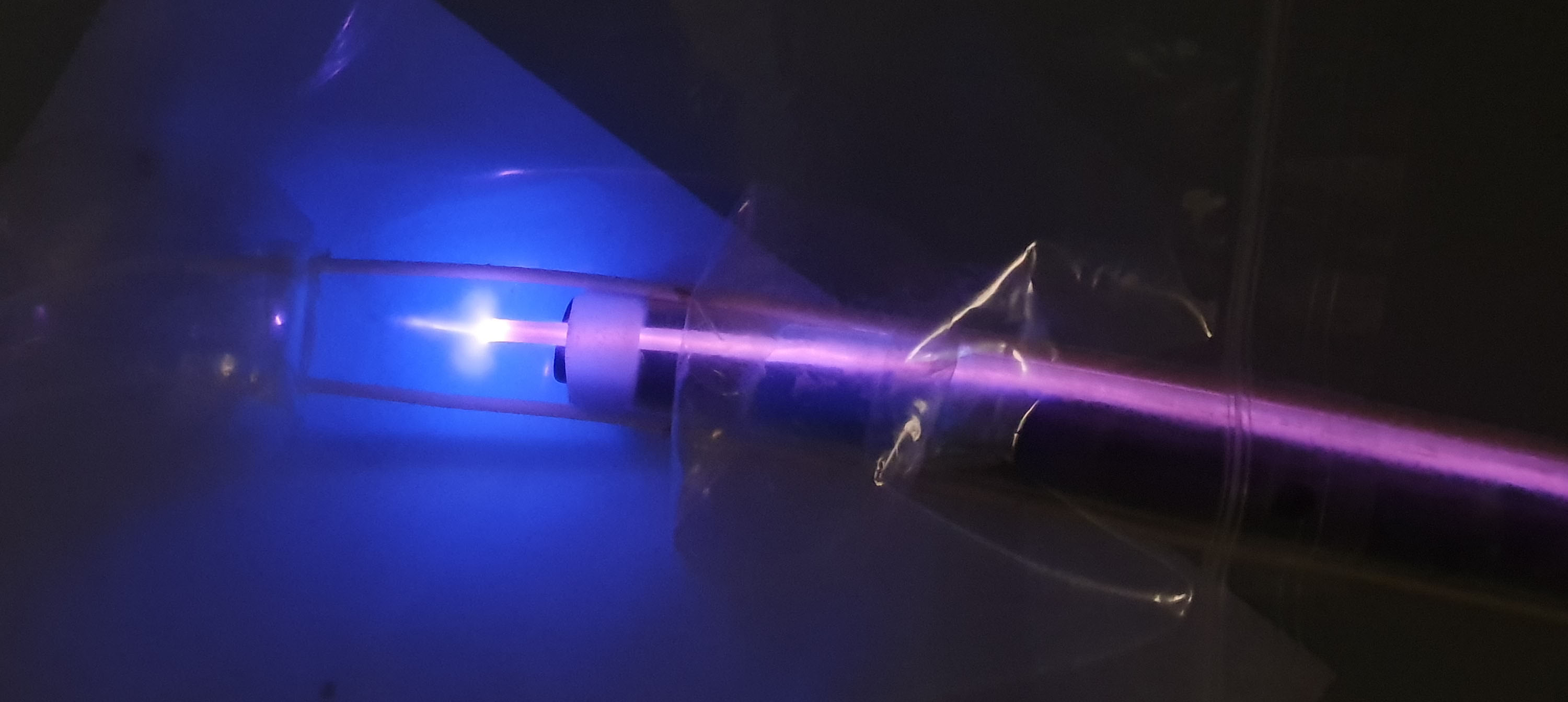
Plasma medicine is an emerging interdisciplinary field; plasma stated as the “fourth state of matter,” is a neutral, partially ionized gas, containing mixture of electrons, photons, atoms, positive and negative ions, radicals, various excited and non-excited molecules. Cold atmospheric plasma (CAP) is an ionized low temperature gas, produced by applying a high voltage electric field at normal or atmospheric pressure. Recently, biomedical applications of CAP have gained great attention because of its promising potential applications such as sterilization, wound healing or blood coagulation, dentistry and tissue regeneration.
Its use in endoscopy is particularly promising, and while a few groups have developed endoscopic plasma systems, they are only suitable for spot treatment in endoscopic surgery, but some medical applications require uniform plasma treatment of larger surfaces, e.g. in the gastro-intestinal tract. In particular, mucosal resurfacing is the ablation of a diseased mucosa followed by a regeneration to a healthy state.
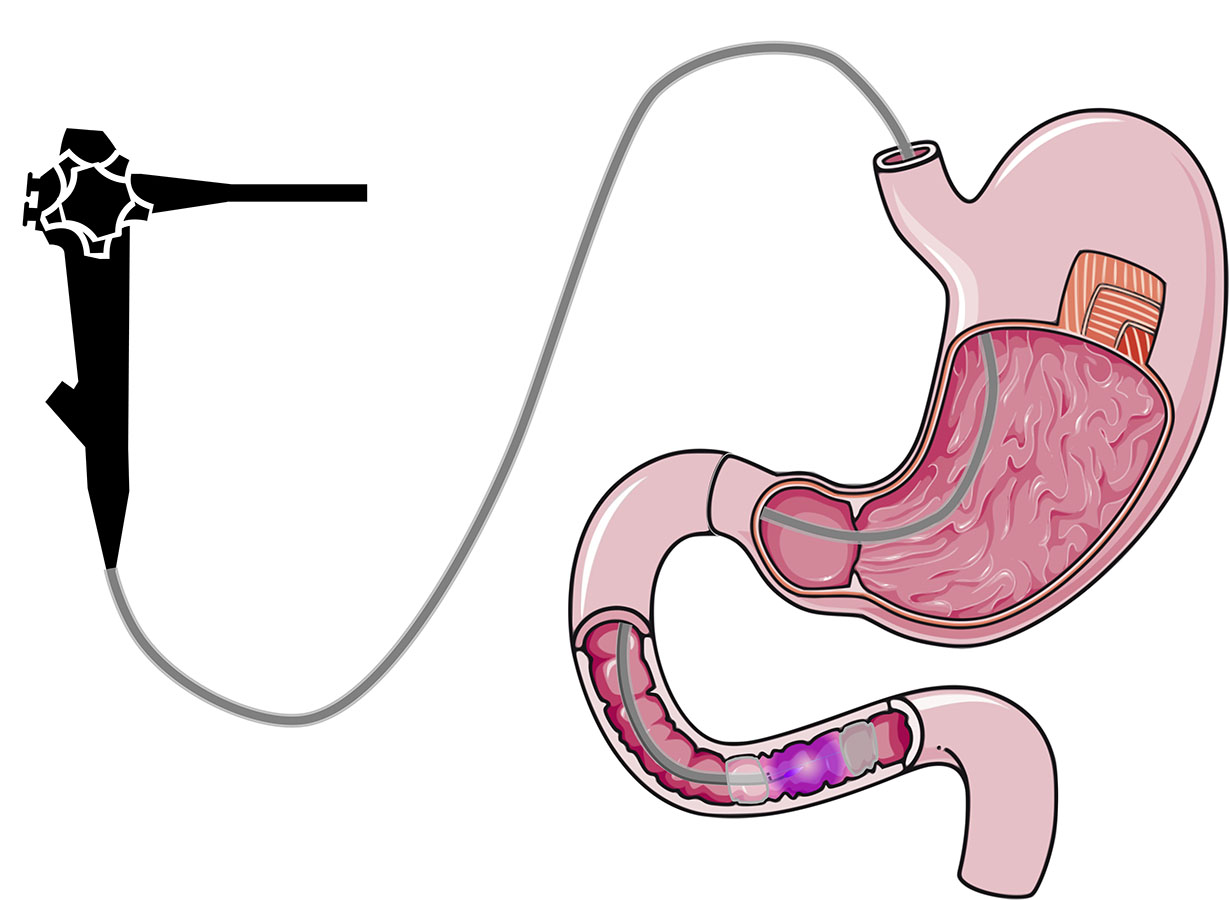
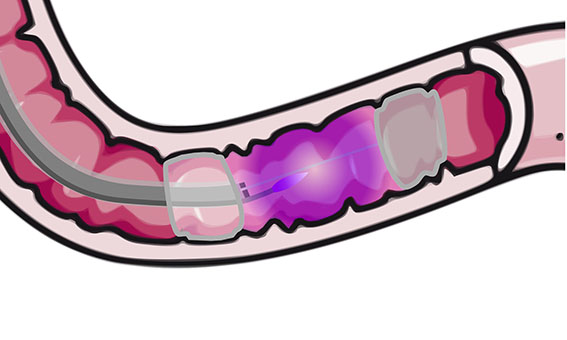
Duodenal Mucosal Resurfacing technique with a new ablation technique using the PHENIX CAP device allows for a quicker, more controlled and homogeneous treatment.
The invention
The objective of the present invention is to provide a device for plasma generation and transport with a high efficiency over a long distance and allowing for a controlled and uniform treatment of large surfaces. The plasma is carried at a long distance from its generation to finally exit at the end of the system to treat efficiently a target area while sparing adjacent areas of tissue with improved control during treatment.
This device is built to perform an ablation of mucosa (i.e. duodenal mucosa) in a minimally invasive way (endoscopically).
Key advantages of the technology
- Homogeneity of treatment.
- No inflammation due to cell-induced death.
- Fast treatment (<1hour).
- Versatile device (i.e. can treat any mucosa of the gastro-intestinal tract).
Commercial interest
- Ablation of the duodenal lining
- Regeneration of mucosa
- Insulin resistance disease (Type 2 Diabetes - TD2) and NonAlcoholic SteatoHepatitis (NASH)
- Barret’s Oesophagus
Technology Readiness Level
The laboratories
It is a truly multidisciplinary project that links various research fields in medicine, engineering, chemistry and biology. The Department of Gastroenterology (LGE) has a large experience and expertise in therapeutic endoscopy, including studies of new devices and mucosal resurfacing.
For several years, the department has developed a successful collaboration with the BEAMS department in developing endoscopic medical devices; the BEAMS department already has substantial experience in minimally invasive devices, and more specifically endoscopic medical devices.
For this specific application, the two groups have partenered with the ChemSIN department, which has a huge expertise in Cold Atmospheric Plasma. This new collaboration is at heart of a field of growing importance: the use of plasma in medicine.
Finally, the IRIBHM biology department, which already collaborates with the LGE in the frame of mucosal resurfacing, brings its expertise on sophisticated 3D cell models so study the action mechanism of CAP on intestinal cells.
Relevant publication
- Optical and Electrical Characteristics of an Endoscopic DBD Plasma Jet, O. Bastin, M Thulliez, J. Servais J, A. Nonclercq, A. Delchambre, A. Hadefi, J. Devière, F. Reniers, submitted to Plasma Medicine, 2020.
Keywords
- Cold Plasma
- Endoscopy
- Resurfacing
Collaboration type
- Licence agreement
- Research cooperation
IP status
- Priority application: EP19153548. 24/01/2019
- PCT application pending.
Contact
ULB Research Department
Arnaud Quintens
Business developer
+32 (0)479 91 22 65
arnaud.quintens@ulb.be