Dans la même rubrique
-
Partager cette page
DPP6 – FXYD2 biomarkers for imaging and targeting Beta-Cells [Offre de technologie]
The technology in a nutshell
New biomarkers expressed in pancreatic beta cells for imaging and targeting beta cells.
State of the art
The progressive decline of beta-cell mass and consequent loss of insulin secretion are key traits in the pathogenesis of autoimmune type 1 diabetes (T1D). Loss and dysfunction of beta cells, caused by metabolic stress, may also contribute to type 2 diabetes (T2D). Variations in the remaining beta cell mass (BCM) among patients are indicative of the disease progression stage (i.e. degree of beta cell loss) and also have an impact on the therapy. An accurate and noninvasive method enabling visualization and quantification of BCM is therefore needed for the development of personalized therapies. BCM information may also be used to evaluate novel pharmaceutical agents that may stop beta cell loss or restore BCM. Beta cells are located in the tiny islets of Langerhans and their mass corresponds to < 2-3% of the total pancreas mass. BMC quantification therefore requires highly specific markers for the development of imaging tracers able to selectively target beta cells located in the pancreatic islets.
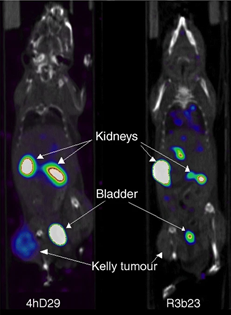
Noninvasive SPECT-CT imaging of Kelly neuroblastoma cells expressing DPP6 in mice using 67Ga-labelled anti-DPP6 4hD29 sdAb vs non-targeting control R3b23 sdAb.
The invention
The present inventions are directed to Dipeptidyl-Peptidase 6 (DPP6) and Sodium/potassium-transporting ATPase gamma chain (FXYD2-y-a), two new human beta-cells islet-specific biomarkers. Those biomarkers, preferentially expressed in beta cells, enabled the development of novel highly specific tracers for non-invasive in vivo imaging of the pancreatic beta cell mass. The biomarkers and corresponding specific probes also make targeted delivery of therapeutic agents, including T regulatory cells, to pancreatic beta cells achievable. These biomarkers provide new tools for diagnosis of diseases characterized by variations in BCM (T1D, T2D, hyperinsulinemia, obesity, insulinomas). They also provide means for treatment monitoring of diabetes, including islet transplantation or attempts at beta cell regeneration.
Specific tracers developed against these targets include a construct of a specific peptide ligand coupled to a contrast agent for magnetic resonance imaging for FXYD2y and specific single domain camelid antibodies (sdAb) for DPP6. Such small sdAbs display unique features with respect to size, stability, and versatility that make them amenable for a wide range of radiolabeling technologies and have already been used for imaging purposes by Single Photon Emission Computed Tomography (SPECT) or Positron Emission Tomography (PET) in animal models of cancer, immunity or atherosclerosis and in the clinic.
Imaging and reliable detection of different amounts of human beta cells implanted into nude mice with the radiolabeled anti-DPP6 sdAb have already been achieved, providing thereby the in vivo proof-of-concept for this technology in animal models. Optimized procedures to label sdAbs with 111In and 67/68Ga isotopes that are respectively suitable for clinical SPECT and PET imaging are also available making the technology ready for a “first in man” trial. The FXYD2 tracer provides on the other hand means for applications with complementary imaging modalities such as MRI.
Key advantages of the technology
- Highly specific human beta-cells islet biomarkers
- Labelled tracers available for non-invasive in vivo imaging of pancreatic beta cell mass by complementary imaging modalities (PET-CT, SPECT-CT, MRI)
- Probes make targeted delivery of therapeutic agents achievable
- Optimized labeling procedures of the tracers readily available
Commercial interest
- Assessment of remaining beta cells in T1D/T2D to allow decision on the best therapies;
- Treatment monitoring of diabetes, including islet transplantation or attempts at beta cell regeneration;
- Development of strategies for targeted delivery of therapeutic agents to pancreatic beta cells.
Technology Readiness Level

PoC demonstrated in a defined animal model
The laboratory
The ULB Center for Diabetes Research investigates the molecular pathways involved in beta-cell impairment and apoptosis in diabetes. For this purpose, members of the laboratory use state of the art methodologies, such as functional genomics, RNA and ATAC sequencing coupled to a systems biology approach, to unveil gene networks regulated by cytokines and free fatty acids.
The inventor
Dr Decio Eizirik is a Professor at the Université libre de Bruxelles (ULB) Center for Diabetes Research (Medical Faculty), Brussels, Belgium. He is also a Senior Investigator at the Indiana Biosciences Research Institute (IBRI) and the Scientific Director of the IBRI Diabetes Center.
He has published more than 385 full papers and reviews in peer-reviewed international journals (h index 85) and has received several national and international prizes, including the Juvenile Diabetes Research Foundation (JDRF) “1998 Diabetes Care Research Award”; the “2012 Albert Renold Prize in Research on Islets of Langerhans” of the European Association for the Study of Diabetes (EASD), and the JDRF “2013 Rumbough Award in T1D research”.
Relevant publication
- Beta Cell Imaging - From Pre-Clinical Validation to First in Man Testing, Int. J. Mol. Sci., 2020, 21, 7274;
- A nanobody-based tracer targeting DPP6 for non-invasive imaging of human pancreatic endocrine cells, Sci Rep, 2017, 7, 15130;
- A nanobody-based nuclear imaging tracer targeting dipeptidyl peptidase 6 to determine the mass of human beta cell grafts in mice, Diabetologia, 2020, 63, 825.
Keywords
- Biomarkers
- Pancreatic beta cells
- In vivo Imaging
- Diabetes T1/T2
- SPECT-PET/CT
- Insulin
- Diagnostic
- Targeted delivery of therapies to beta cells
Collaboration type
- Licence agreement
- R&D&I collaboration
IP status
- DPP6 - priority 22/04/2016 - Patent application pending - US2019145987 ; EP3446126;
- FXYD2 - priority 14/02/2008 - Patent Granted US8425878; JP5697995; EP2247717 (BE, CH, DE, FR, GB).
Main inventor
Contact
ULB Research Department
Fred Pierard
IP Manager
+32 (0)2 650 32 26
frederic.pierard@ulb.be